DeepQure Gets Green Light for HyperQure™ RDM System Clinical Trial in South Korea
DeepQure has announced a significant step forward in their medical technology development. South Korea’s Ministry of Food and Drug Safety (MFDS) has officially approved a clinical trial for their HyperQure™ RDM System. This approval marks a crucial milestone for the company and potentially for the future of related treatments.
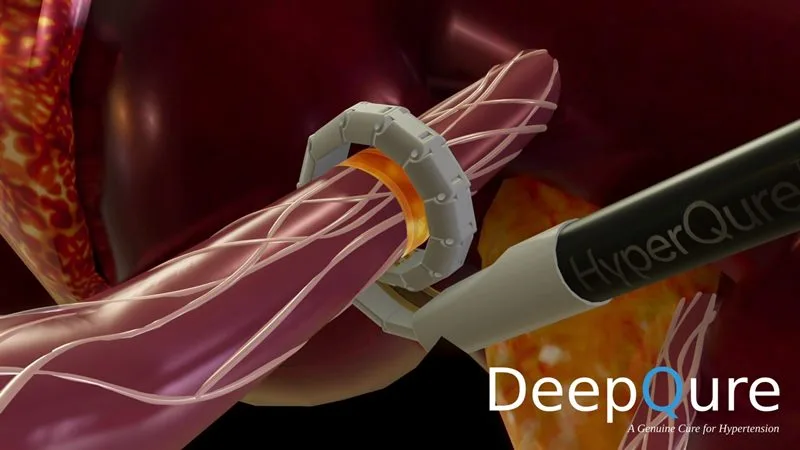
What is HyperQure™ RDM System?
While details are still emerging, the HyperQure™ RDM System appears to be an innovative medical device. The clinical trial will provide vital data on its safety and efficacy. The specifics of the system’s function and target condition are anticipated to be released as the trial progresses.
Significance of MFDS Approval
The MFDS approval is a rigorous process, indicating that the HyperQure™ RDM System has met certain preliminary standards. This clearance allows DeepQure to proceed with human trials in South Korea, gathering essential data for potential future regulatory approvals and commercialization.
Looking Ahead
The upcoming clinical trial will be closely watched by the medical community and investors alike. The results will determine the future path of the HyperQure™ RDM System and its potential impact on patient care.
- Clinical trials will evaluate safety and efficacy.
- Success could lead to wider availability.
- More information is expected as the trial advances.
Final Words
DeepQure’s approval for a clinical trial in South Korea represents a pivotal moment for the company and highlights the ongoing advancements in medical technology. The results of this trial will be instrumental in determining the future of the HyperQure™ RDM System and its potential benefits for patients.


+ There are no comments
Add yours