Promising Lyme Disease Vaccine Shows Positive Results in Clinical Trials
A novel Lyme disease vaccine, known as VLA15, is demonstrating encouraging safety and effectiveness in clinical trials. The investigational vaccine has shown a favorable safety profile and the ability to stimulate a strong immune response in participants of all ages, with children and adolescents exhibiting particularly robust responses. This news offers hope for a potential preventative measure against Lyme disease, a growing concern in many parts of the world.
Key Findings from the VLA15 Vaccine Trials
The clinical trials have provided valuable insights into the VLA15 vaccine’s performance:
- Safety: The vaccine has been well-tolerated across all age groups, indicating a promising safety profile.
- Immunogenicity: VLA15 has proven effective in eliciting a strong immune response, suggesting it can provide protection against Lyme disease.
- Age-Related Response: Children and adolescents showed a particularly strong immune response, which is significant given their higher risk of exposure in certain regions.
Implications for Lyme Disease Prevention
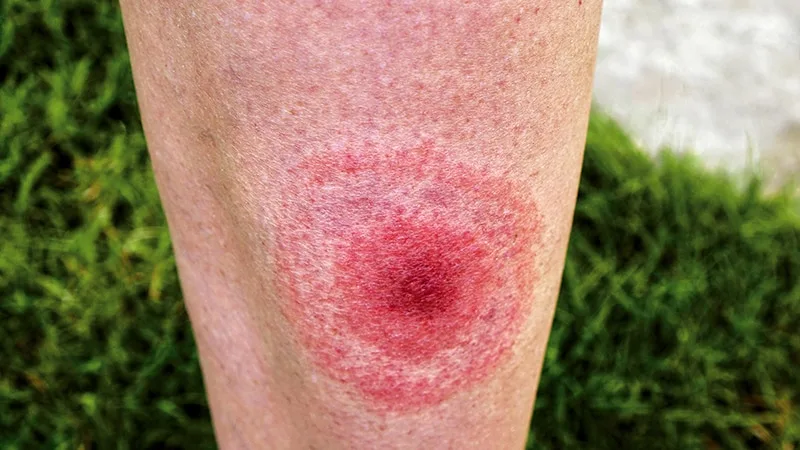
Lyme disease is a bacterial infection transmitted through the bite of infected blacklegged ticks. Early symptoms can include fever, headache, fatigue, and a characteristic skin rash called erythema migrans. If left untreated, the infection can spread to the joints, heart, and nervous system.
The potential availability of a safe and effective Lyme disease vaccine could significantly reduce the incidence of this debilitating disease, especially in high-risk populations.
Future Directions for VLA15
While the initial trial results are promising, further research and development are necessary to fully evaluate the long-term efficacy and safety of the VLA15 vaccine. Larger-scale Phase 3 trials will be crucial to confirm these findings and pave the way for potential regulatory approval.
The Road Ahead
- Continued monitoring of participants in the clinical trials.
- Conducting Phase 3 trials to assess long-term efficacy.
- Evaluating the vaccine’s effectiveness against different strains of Lyme disease.
Final Overview
The development of the VLA15 Lyme disease vaccine represents a significant step forward in the fight against this common tick-borne illness. The favorable safety profile and strong immunogenicity observed in clinical trials provide hope for a future where Lyme disease can be effectively prevented, safeguarding the health of individuals of all ages.


+ There are no comments
Add yours