Darmiyan Inc. Receives FDA Approval for Brain Health Technology
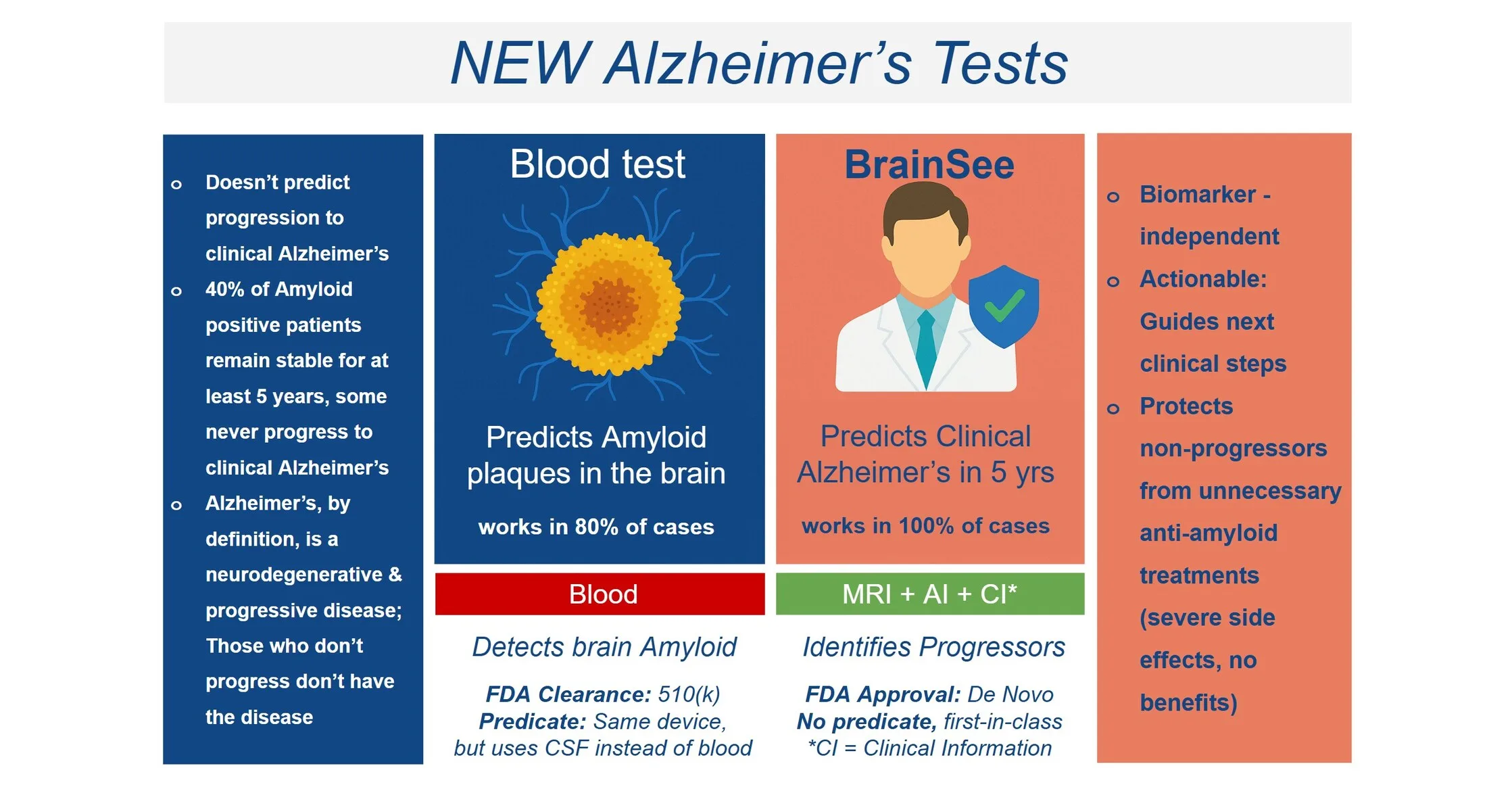
Darmiyan, Inc., a prominent innovator in brain health technology, has announced a significant milestone. The U.S. Food and Drug Administration (FDA) has granted approval for their novel technology, marking a pivotal moment for the company and the broader field of brain health diagnostics.
Impact on Brain Health Diagnostics
This FDA approval paves the way for enhanced diagnostic capabilities, potentially leading to earlier and more accurate detection of various brain health conditions. Darmiyan’s technology aims to provide clinicians with advanced tools to assess and monitor brain function, ultimately improving patient outcomes.
Key Benefits of the Approved Technology:
- Enhanced accuracy in brain health assessments
- Potential for earlier detection of neurological conditions
- Improved monitoring of treatment efficacy
The Future of Brain Health Technology
The approval underscores the growing importance of technological advancements in healthcare. As brain-related disorders continue to pose significant challenges, innovative solutions like Darmiyan’s technology offer hope for better management and treatment strategies.
Broader Implications:
- Encourages further research and development in brain health technologies
- May lead to the development of personalized treatment approaches
- Could reduce the burden of neurological diseases on individuals and healthcare systems
Final Words
With this FDA approval, Darmiyan, Inc. is poised to make a substantial contribution to the field of brain health. The technology holds promise for transforming diagnostics and ultimately enhancing the lives of individuals affected by neurological conditions. This milestone reflects the ongoing progress in medical technology and its potential to address critical healthcare needs.

+ There are no comments
Add yours