The World Health Organization (WHO) has approved Bavarian Nordic’s mpox vaccine, Jynneos, for adolescents aged 12 to 17. This age group is considered especially vulnerable to mpox outbreaks, which have raised global concerns. The vaccine was prequalified by WHO on October 8, marking a crucial step in expanding access to this preventive measure.
Mpox Outbreak and WHO’s Actions
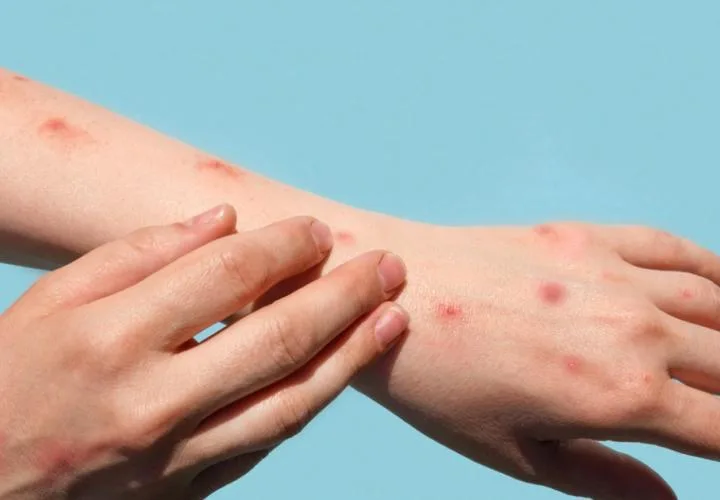
In August, WHO declared mpox a global public health emergency for the second consecutive year after a new strain of the virus spread from the Democratic Republic of Congo to neighboring countries. This viral infection primarily causes flu-like symptoms and pus-filled skin lesions, with children, adolescents, and immunocompromised individuals being particularly at risk.
In September, WHO approved the use of the Jynneos vaccine for adults, providing easier access to the shot in African countries, where mpox cases have been prevalent. Now, with the extension to adolescents, WHO aims to further protect those who are most vulnerable to the virus.
Expanding Research for Younger Age Groups
Bavarian Nordic, the Danish biotech company behind the vaccine, is also preparing to conduct a clinical trial to assess the vaccine’s safety in children aged 2 to 12. The trial is partially funded by the Coalition for Epidemic Preparedness Innovations and is expected to begin in October.
Global Vaccine Approvals
The United States Food and Drug Administration (FDA) has approved Bavarian Nordic’s mpox vaccine for adults aged 18 and older, but it granted Emergency Use Authorization for adolescents during the mpox outbreak in 2022. Additionally, the European Union approved the vaccine for adolescents in September, further expanding its global reach.
Japan’s KM Biologics also produces an mpox vaccine, LC16, which can be administered to children but requires a specialized needle for proper delivery, according to the Japanese regulator.
Global Mpox Concerns
New mpox cases continue to emerge globally, with confirmed infections of a new strain reported in countries like the Democratic Republic of Congo, Sweden, Thailand, Burundi, Kenya, Rwanda, and Uganda.
The approval of the vaccine for adolescents is a key step in preventing the spread of mpox, especially among young people, and addressing the rising concerns around this viral infection.
MpoxVaccine #WHOApproval #AdolescentHealth #BavarianNordic #GlobalHealthCrisis #MpoxPrevention



+ There are no comments
Add yours